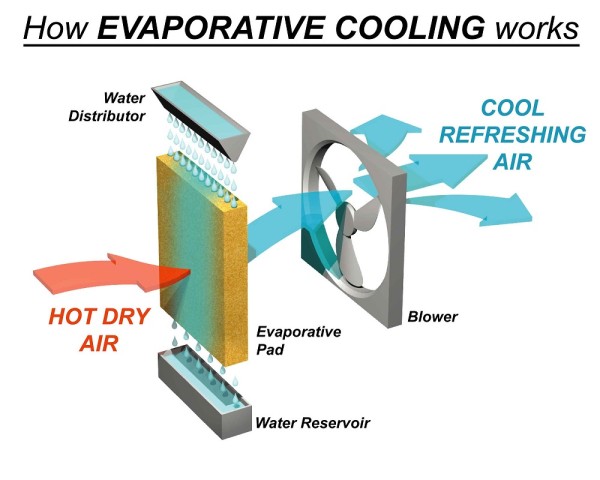
Evaporative Air Cooling Principle
Evaporative cooling is the natural way of cooling, similar to a breeze flowing across a lake. The air temperature is lowered due to its contact with the water and has a relaxing, cooling effect on people. An evaporative cooler cools the air by means of the evaporation of water. When water evaporates into the air, the result is a state change of water from liquid to gas molecules. This chemical change requires heat, thus energy, or latent heat, is taken from the warm air molecules creating a drop in the air temperature.
When hot air enters the cooling pads evaporation occurs i.e., hot air absorbs the cool water molecules present in cooling pads and the cool breeze enters the room through the fan.
Small distribution lines supply water to the top of the pads. Water soaks the pads and, thanks to gravity, trickles through them to collect in a sump at the bottom of the cooler. A small re-circulating water pump sends the collected water back to the top of the pads.
Since water is continually lost through evaporation, a float valve – much like the one that controls the water in a toilet tank – adds water to the sump when the level gets low. Under normal conditions, a swamp cooler can use between 3 to 15 gallons of water a day.
A large fan draws air through the pads, where evaporation drops the temperature approximately 20 degrees. The fan then blows this cooled air into the house.
“If we want to move towards a low-polluting, sustainable society, we need to get consumers to think about their purchases.”